
Unique Periodic Table song Hydrogen Helium Lithium Beryllium tablepriodic priodic
The atomic number of an element provides insight into the number of protons that exist inside the nuclei of the atoms of that element and also into the number of electrons that surround these nuclei. For example, the atomic number of sodium is 11.
Science online The electronic configuration and the chemical activity
5: The Electronic Structure of Atoms
Why does hydrogen naturally form diatomic molecules (H2), whereas helium exists as a single atom
5.14: Hydrogen, Helium, Lithium. With some familiarity with the properties of single electrons, such as the single electron around the hydrogen nucleus above, we can discuss atoms containing more than one electron. The images found here depict electron wave density by number of dots. Thus, more dots indicates more electron density 'cloud' in.

Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B DocsLib
Hydrogen. Helium. Lithium. Oxygen. Carbon. Nitrogen. Neon. Magnesium. Silicon. Sulfur. Iron. Aluminum. Calcium. Argon. Sodium. Krypton. Xenon. Barium. Strontium. JPEG screen grabs of an applet which computes and plots the spectra in a web browser window. The above images aren't dithered. They may appear so if your display doesn't have enough.

Periodic Table Hydrogen Periodic Table Timeline
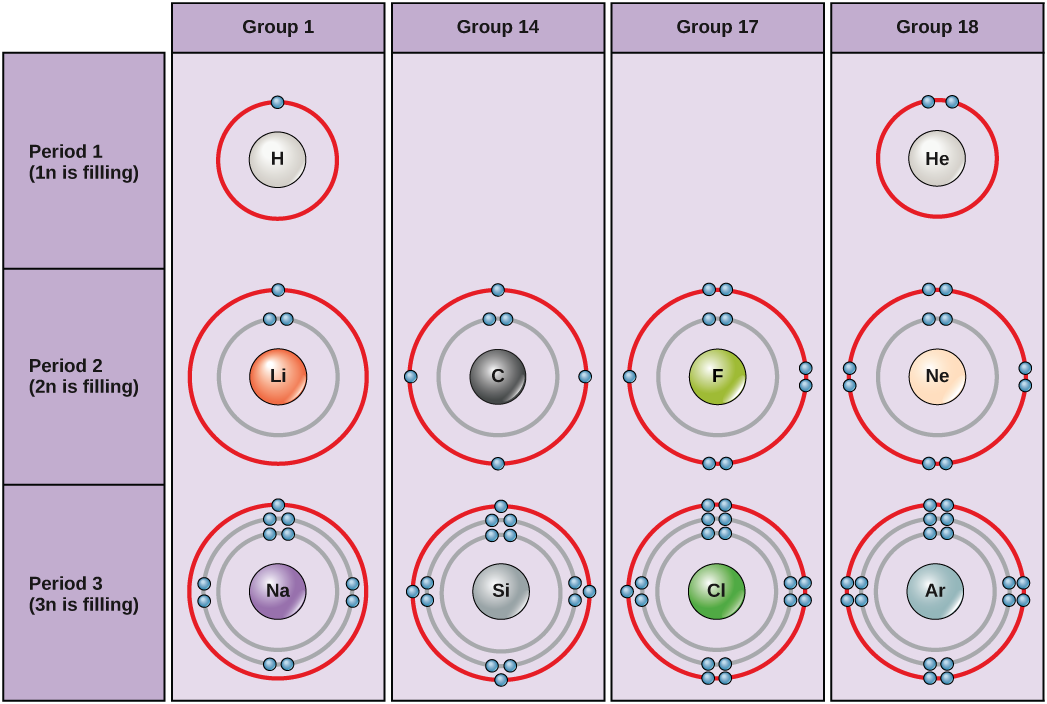
Bohr diagrams for hydrogen, helium, lithium, carbon, fluorine, neon, sodium, silicon, chlorine, and argon. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.

Hydrogen, Helium, Lithium Loops Set Stock Motion Graphics Motion Array
Figure 6.19.1 6.19. 1 The figures above show the electron density of different elements. On the left from top to bottom are Hydrogen, Helium, and Lithium. On the right from top to bottom are Beryllium, Boron, and Carbon. Hydrogen has a larger circular area concentrated with dots when compared to helium.

hydrogen helium lithium beryllium... Drawception
Li = 75:62eV for helium and lithium, respec-tively. For the ionization of the third lithium electron an energy of I(3) Li = 122:42eV is required. For full thermal ionization of all helium or lithium electrons we need far more than 100eV, i.e. more than 106 K. An alternative way to reach high ionization degrees is to increase the particle

Unique Periodic Table song Hydrogen Helium Lithium Beryllium tablepriodic priodic
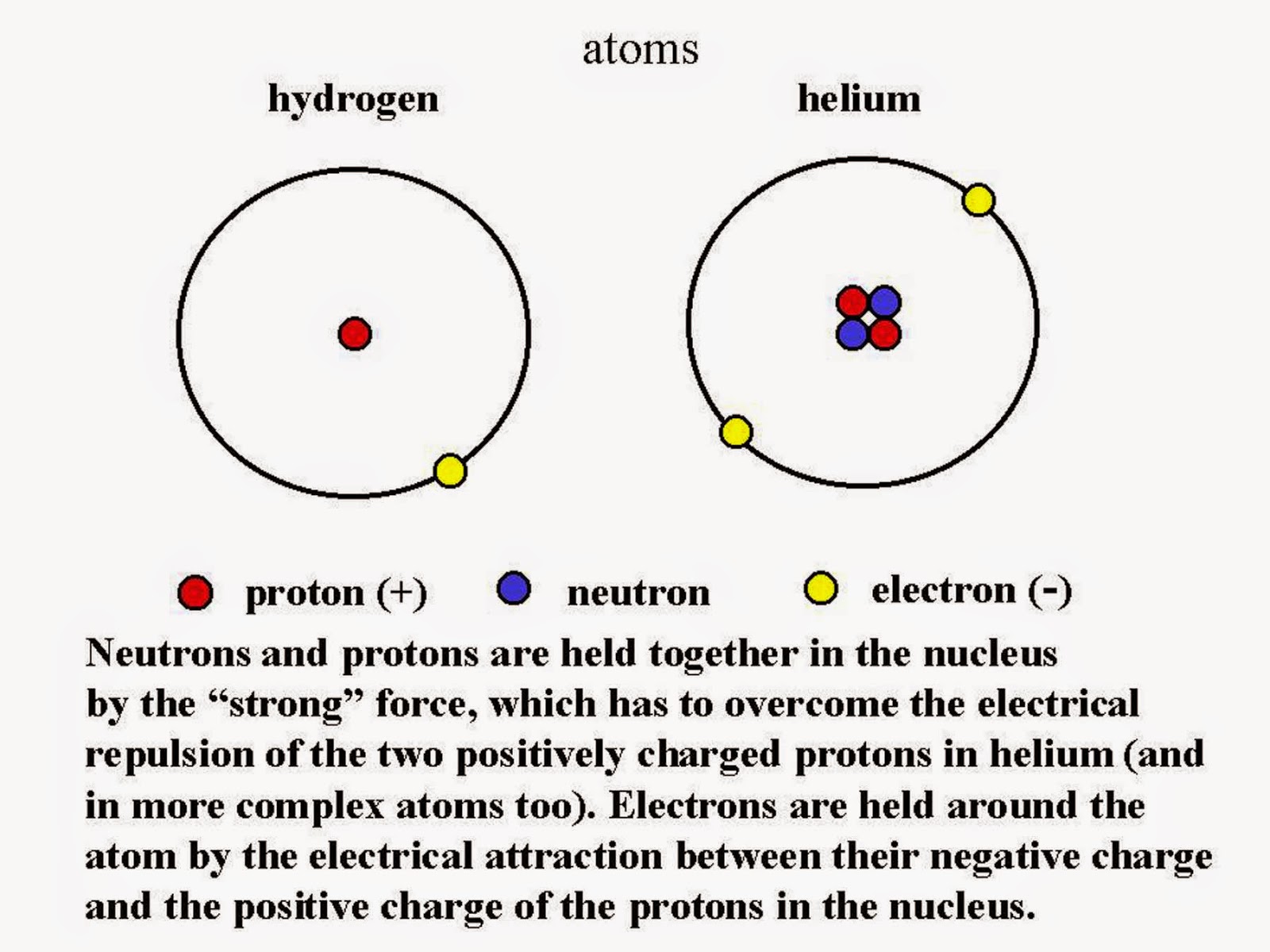
Example 1: Helium vs. Lithium. Hydrogen has an electronic structure of 1s 1. It is a very small atom, and the single electron is close to the nucleus and therefore strongly attracted. There are no electrons screening it from the nucleus and so the ionization energy is high (1310 kJ mol-1). Helium has a structure 1s 2. The electron is being.
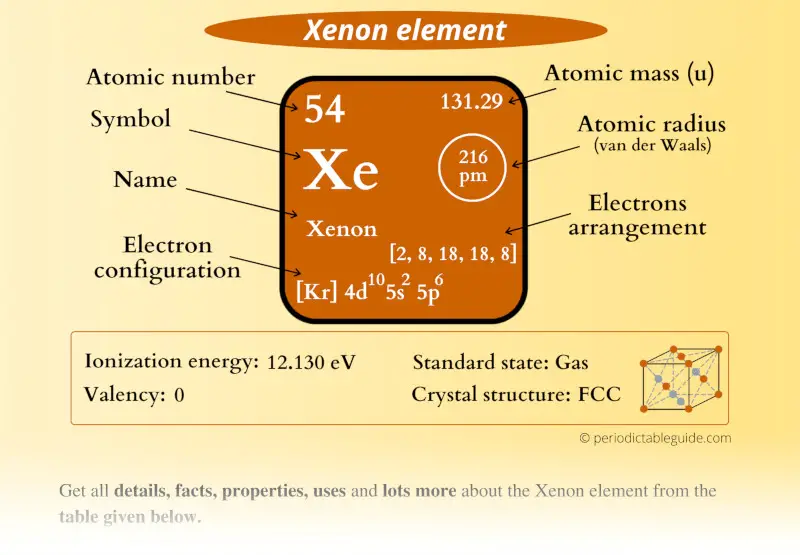
Xenon (Xe) Periodic Table (Element Information & More)
Lithium is rare in the Universe, although it was one of the three elements, along with hydrogen and helium, to be created in the Big Bang. The element was discovered on Earth in 1817 by Johan August Arfvedson (1792-1841) in Stockholm when he investigated petalite, one of the first lithium minerals to be discovered.
Elektronen Biologie für Hauptfach I Great Journey
for allowed and forbidden lines of hydrogen, helium and lithium, including Li II, as well as the hydrogen isotopes deuterium and tritium. Altogether, we tabulated about 3600 transitions and listed scaling relations for the hydrogenlike ions He II and Li III. The selected data are based on a critical evaluation of available literature sources.

Table Périodique D'éléments Fondamentaux Illustration Stock Illustration du azote, actif 6527575
nuclear astrophysics High-energy nuclear physics Scientists Physics portal Category v t e Nuclear fusion is a reaction in which two or more atomic nuclei, usually deuterium and tritium (hydrogen variants), combine to form one or more different atomic nuclei and subatomic particles ( neutrons or protons ).

Hydrogen, helium and lithium atoms, illustration Stock Image C050/7595 Science Photo Library
Therefore, only hydrogen, helium and lithium were available when the earliest stars formed several hundred million years later. As we have seen, almost all of this material was 1 H and 4 He. The reactions that could form the lightest elements beyond hydrogen and helium are shown in Figure 16.8. The formation of beryllium is speculative, as we.
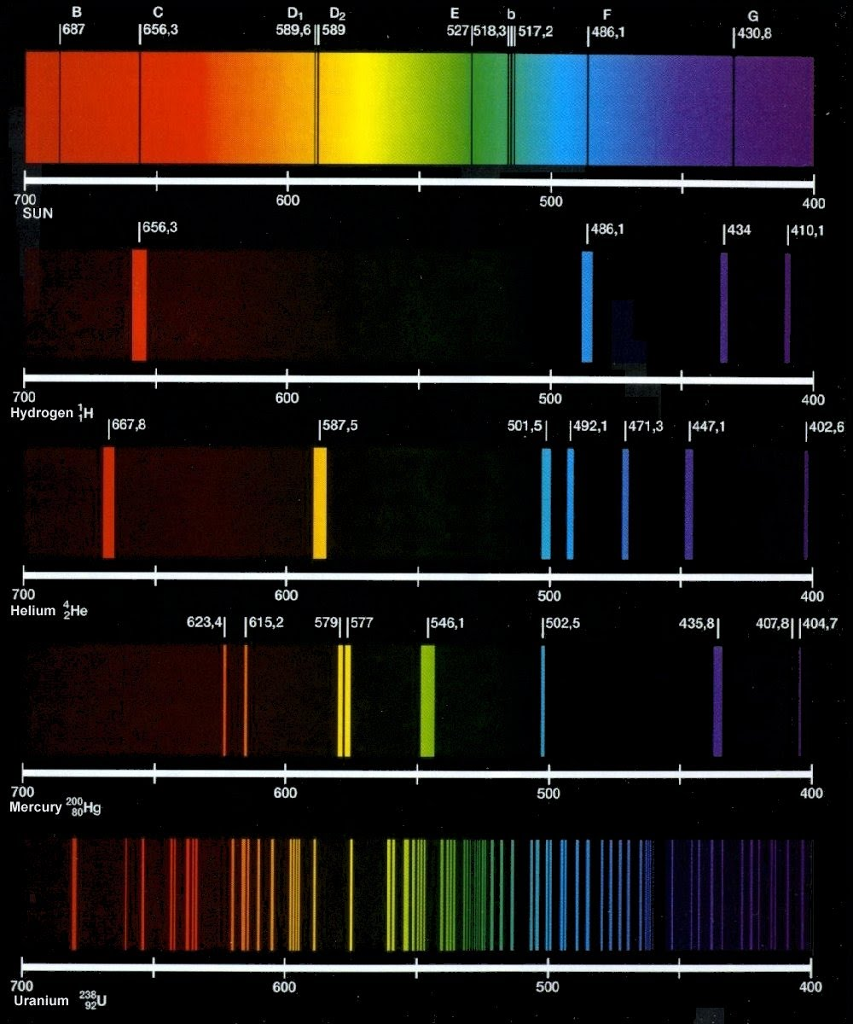
Solved Di D2 589,6 1589 656,3 527518311517,2 1486,1 430,8
The periodic table is a chart that organizes the elements by their atomic number. The first 30 elements are hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon, sodium, magnesium, aluminum, silicon, and phosphorus.
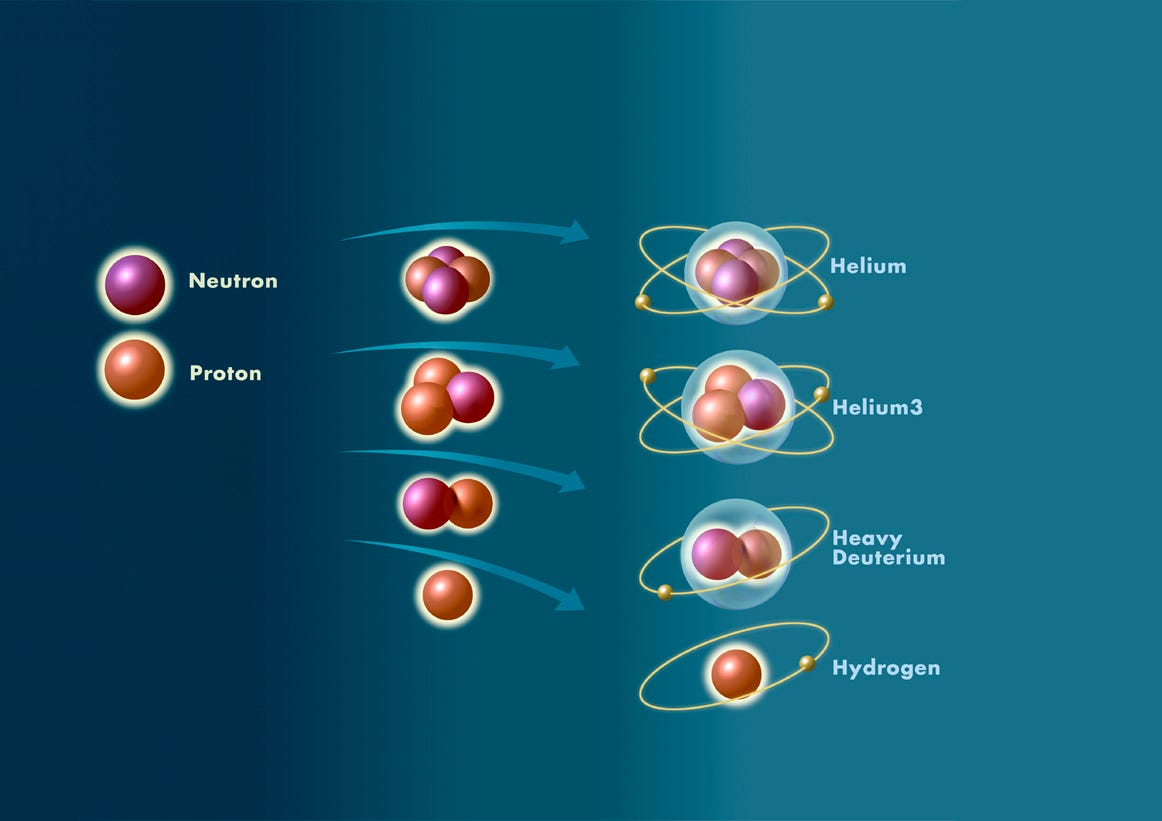
Why did the Universe start off with Hydrogen, Helium, and not much else?
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. Electron configuration of Hydrogen (H) 1s 1: 1s 1: 1: 2: Electron configuration of Helium (He) 1s 2: 1s 2: 2: 3: Electron configuration of Lithium (Li) [He] 2s 1: 1s 2 2s 1: 2, 1: 4:

The H2eVTOL Council's Pioneering Year
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.

Atomic structure of Hydrogen, Helium,Lithium,Beryllium,Boron,Carbon,Nitrogen,Oxygen,Fluorine
Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent.